A grant of nearly $2.8 million awarded to University of Nebraska-Lincoln professor Steven Barlow will be used to study the link between the brain’s molecular pathways and the development of oral feeding skills in extremely preterm infants.
Barlow, Corwin Moore Professor in UNL’s Department of Special Education and Communication Disorders, will lead the five-year, multi-site study that will examine 180 preterm infants born between 24-27 weeks at neonatal intensive care units at CHI Health St. Elizabeth in Lincoln, Tufts Medical Center in Boston and Santa Clara Valley Medical Center in San Jose, California. The group of about 20 researchers will include neonatologists, geneticists and nurses at all three medical centers, as well as small groups at Texas Tech University and Harvard University.
Babies must be able to take all feedings by nursing, bottle or a combination before they can be discharged from the NICU. The research by Barlow’s team aims to shorten the amount of time infants have to remain in the NICU.
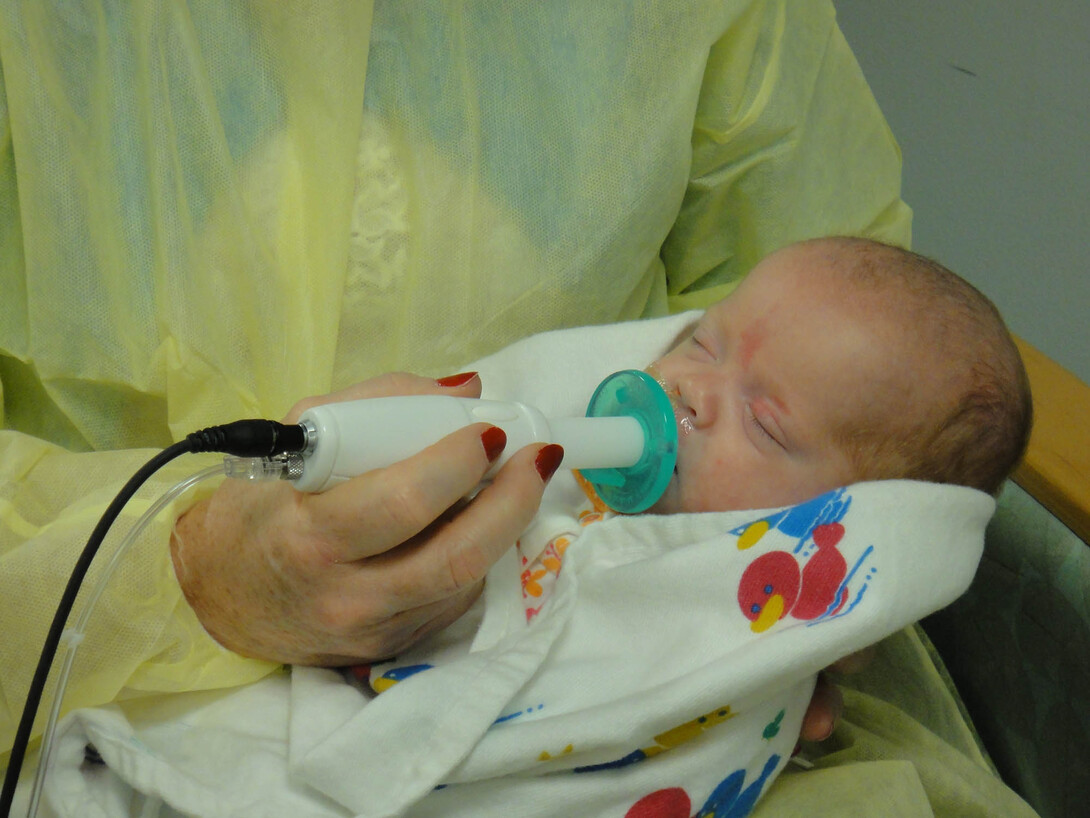
Previous research has examined the changes in babies’ feeding behaviors using the NTrainer System, a therapy device developed by Barlow and approved by the Food and Drug Administration in 2008, to stimulate early development of essential feeding skills in preterm infants. This new study by Barlow, funded by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health, will be the first to explore the ability of the NTrainer’s stimulation to trigger positive genetic changes. While in the NICU, some infants in the study will be randomized to use the NTrainer System to generate a pulsating oral touch stimulation to the lips and tongue during tube feedings, while others will receive a sham, which is a typical non-pressurized pacifier.
“What is being hypothesized is that the three-times-a-day stimulation from the NTrainer is going to accelerate what these target genes do,” Barlow said. “If we can demonstrate that, then we’ve uncovered a new therapeutic approach that will provide neonatologists and feed therapists with the knowledge to formulate individualized oral feed advancement therapies based on the infant’s neurobiology.”
The researchers will collect a droplet of saliva from the infants every three days while they are in the NICU. Those samples will be frozen and shipped to Tufts for processing. There, the saliva samples will undergo reverse transcription polymerase chain reaction (RT-PCR), which creates a mirror image of the DNA that will allow the researchers to observe any changes in six target genes. Each of the six genes relate to different aspects of feeding, including hunger, satiation, oral touch sense, muscles in the feeding mechanism, and migration and pathway formation of the developing neurons from the brain stem outward. Researchers will look at the change in protein levels within those genes to determine the impacts of the NTrainer therapy.
When the infants are between 18-24 months old, they will undergo follow-up testing at the three NICUs to examine their growth and development. Part of this follow-up will include the Bayley-III Screening Test, which assesses cognitive, language and motor skills. The statistics and data from the infants who received the NTrainer therapy will then be compared to those who received the sham to identify the impacts of the NTrainer on the infants’ neurodevelopmental outcome.
While this research is being conducted on extremely preterm infants, Barlow hopes the findings will be applicable to people of all ages.
“These are the most fragile babies on the planet, and the technologies that are developed with this population will probably end up benefitting a lot of other child populations,” Barlow said. “We are thinking it may benefit people at the other end of the lifespan too, including survivors of traumatic brain injuries and stroke. A lot of those patients have to relearn feeding and speaking, and these types of therapies will hopefully be beneficial.”
The National Institutes of Health award number is R01HD086088. The content of this news release is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.










